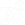The main challenge in preclinical oncology is the translation of human cancer into the animal models. Recent technological revolution has made possible the generation of genetically engineered mouse models. The genetic profile of these mice is altered in order to develop de novo tumors in a natural immune-proficient microenvironment. The developed tumors closely mimic the genetic composition as well as the drug response, and resistance observed in human cancer. However, the targeted gene modifications cannot fully simulate the complexity of the human tumor, while it is also a costly model, requiring years of work.
In contrast to GEM models, the human tumor xenograft is widely used, allowing rapid analysis of human tumor response to a specific therapeutic regime in vivo. In the ectopic tumor xenograft model (or subcutaneous xenograft model), an established human cancer cell line is subcutaneously injected into the hind leg or back of immunocompromised mice e.g., athymic nude mice (nu/nu) or severe combined immune-deficient mice (scid/scid) (Fig.2.5.5). Anticancer activity can be evaluated by easily monitoring tumor growth. Hence, this model is very reproducible and amenable to use, but it cannot be applied to tumors that show necrosis or are not solid.

Another type of animal model to test anti-tumor compounds is the orthotopic tumor xenograft model, in which the human cancer cells are engrafted into the relevant organ of tumor origin (Fig.2.5.5). As cancer cell lines used in this model express fluorescence or luciferase, the tumor growth can be monitored by optical imaging, computerized tomography (CT) or magnetic resonance imaging (MRI). In contrast to the subcutaneous xenograft model, the orthotopic tumor model is more clinically relevant owing to the establishment of an organ-specific tumor microenvironment, which can differ in each cancer type. However, surgical skills as well as specialized expensive equipment are required to monitor tumor progression and metastatic activity.
Since the xenograft models using cancer cell lines are not sufficient to represent the complex tumor heterogeneity, patient-derived xenograft (PDX) models have emerged. The establishment of a PDX is accomplished by transplanting freshly isolated tumors from patients into immunocompromised mice. This personalized approach, which preserves the heterogeneity of the original tumor, is a powerful tool because of its predictive therapeutic value. On the other hand, these models have technical constraints, and are expensive and time-consuming.
Moreover, PDX models cannot completely recapitulate the human tumor immune microenvironment; thus, humanized mice have been developed by engrafting cultured CD34+ hematopoietic stem cells (HPCs) or cultured precursor cells (HSPCs) that resemble better the human immune system. Improved humanized mice have been generated by combining the humanized mouse with either a human tumor xenograft or a patient-derived xenograft (Fig. 2.5.6) .